Colloidal Silver; What is it Really?
Colloidal Silver (Ag) is a solution of extremely fine sub microscopic particles (.001 - .005 microns)
of pure silver suspended in water by a positive electric charge on each particle. The particles remain suspended throughout the solution because these positive charged particles repel each
other.
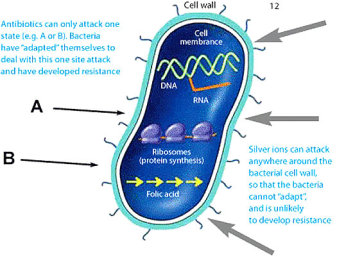
A powerful germicidal, silver is an exceptional metal in that it is non-toxic to the human body, but lethal to over 650 disease causing bacteria, viruses, fungi, parasites and molds; while conventional pharmaceutical antibiotics are typically effective against only 6 or 7 types of bacteria. Some new strains of bacteria classified as MDR (Multiple Drug Resistant) have proven to be resistant to all pharmaceutical antibiotics, but not to colloidal silver due to different germicidal mechanisms of deactivation.
How does colloidal silver work?
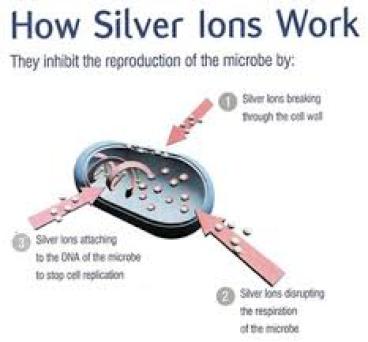
Richard Davies and Samuel Etris of The Silver Institute, in a 1996 report entitled The Development and Functions of Silver in Water Purification and Disease Control, discuss three
primary ways that silver deactivates disease causing organisms.
Catalytic Oxidation:
Silver naturally holds onto oxygen molecules, which readily react with the sulfhydral (H) groups
that surround bacterial and viruses. In turn, this helps block the life-preserving cellular
process known as cellular respiration, which is defined as “the set of metabolic reactions and
processes that take place in the cells of organisms to convert biochemical energy from nutrients
into adenosine triphosphate (ATP), and then release waste products.” In sum, producing oxidation.
Reaction with Bacterial Cell Membranes:
Silver ions can attach to bacterial cell membranes directly, impairing cell respiration and blocking its energy transfer system.
Binding with DNA:
Shown to literally enter bacteria DNA, up to 12% of silver has been detected in Pseudomonas Aeruginosa. According to one source, “While it remains unclear exactly how the silver binds to the DNA without destroying the hydrogen bonds holding the lattice together, it nevertheless prevents the DNA from unwinding, an essential step for cellular replication to occur.”
Colloidal or Ionic?
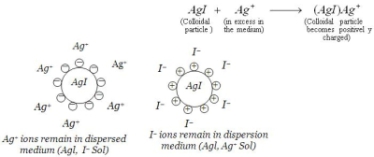
The word "colloidal" refers to a condition where, in this case, a solid particle is SUSPENDED in a liquid (silver in water). The solid particles are too large to be considered DISSOLVED,
but are too small to be filtered out. This colloidal condition is most easily detected by what is called the "Tyndall effect", where a narrow beam of light is shined through the liquid to
produce a cone shaped dispersion of the light. The particles so illuminated also exhibit a random, zigzag activity called "Brownian motion" when observed under a microscope. When something
is completely dissolved, both the Brownian and Tyndall effects disappear.
The word "ionic" refers to a condition where a particle has an electric charge. In the case of colloidal silver, this electric charge is ALWAYS positive. Silver will not form a negatively charged ion. So, colloidal silver is BOTH colloidal and ionic. It is considered colloidal because of the particle SIZE and it is considered ionic because of the particle CHARGE. In fact, most of the biological studies suggest it is colloidal silver's ionic characteristics that make it such a good germicide. It is also interesting to note that the old chemistry books make no distinction between the colloidal and ionic states of the electro-colloidal metals.
The Test:
There is considerable discussion and controversy regarding whether ionic silver or particulate (also known as true) colloidal silver is more efficacious.
It is claimed by one manufacturer that colloids are more effective at killing pathogens. Their product is about 90% colloidal while most ionic/colloidal silver is typically 75-95% ionic. They claim that ionic silver is converted to silver chloride in the stomach by coming in contact with hydrochloric acid, thereby becoming ineffective.
Another manufacturer claims that the ionic portion is more effective at killing pathogens. Their product is about 95% ionic.
In order to resolve the dispute, kill studies were performed using a controlled comparison that diluted both colloidal silver products with hydrochloric acid to neutralize the ions in the first manufacturers product. Then the two were diluted to 20 PPM each so the comparison would have validity. They then combined the remaining fractions of the two products with two strains of Staphylococcus Aureus. The dilutions were placed on petri plates and graded as to their effectiveness in killing the pathogens.
Please see the test results here.
**This should resolve the issue.
It seems clear the ionic portion is more effective at killing pathogens.
(Check out the youtube video at the bottom of the "About Production" page)
Effectiveness (Efficacy)
Colloidal silver's effectiveness as a broad-spectrum germicide is directly related to the number
and size of the particles. The same volume of space taken up by one silver particle which is
.1 microns in size, will hold about 10,000 silver particles that are .001 microns in size. This reduction in particle size not only allows for a greater distribution of the silver, but it also greatly increases the total surface area of silver available for interacting with microbes. These, plus the stability of the electrical charge, are the most important factors when considering the effectiveness of colloidal silver.
PPM : IS MORE PPM REALLY BETTER?
The concentration of silver in the water is usually measured in parts per million, or ppm.
While this is the standard convention, ppm is a "ratio" and not an indicator of quantity.
When a laboratory tests colloidal silver for concentration, they report the findings in
milligrams per liter (mg/L). Milligrams per liter is an actual measurement of weight per volume, and therefore is a real quantity measurement. In the metric system, one liter of
water weighs 1000 grams, and one milligram is one thousandth (1/1000) of a gram,
so 1 mg/L is the same as 1 ppm, as long as we are talking about water. Silver weighs a little more than water, but the equivalence is very close, and the terms are often used interchangeably. With this in mind, we can calculate that one teaspoon of 5 ppm colloidal silver has about 25 mcg (micrograms) of silver in it.
A Good Example: If one were to ingest that 1 mg. piece of silver, it would not do
much good. If you were to divide that 1 mg. into 10,000 particles of silver, it would then have much more efficacy because many more micro particles can come into direct contact with
pathogens. The silver is now 10,000 times better than 1 piece of silver even though by definition it is still only 1 mg or 1 PPM. So, what really counts is not necessarily how many PPM the silver is, but how many micro particles the silver has been divided into.
Purity of Silver
The quality of your finished product depends entirely on the purity of the water you start with and the purity of the silver you start with. Most of the current literature suggests that only 99.999% pure silver can be used. Most home brew systems use 99.9% pure silver. So, what is the difference?
According to Academy Metals, a company in Albuquerque, New Mexico, that produces commercial silver, the total allowable impurities in 99.9% (.999 fine) silver is 1000 ppm or 1 part in 1000. These impurities and their maximums are 1) Copper, 800 ppm, 2) Lead, 250 ppm, 3) Iron, 200 ppm, and 4) Bismuth, 10 ppm. This product is readily available in wire form and costs about $3.00 above the market (spot) price of silver. When this product is used to make electro-colloidal silver at a concentration of 5 ppm, the total impurities from the silver drop to 4 ppb (parts per billion) copper, 1.25 ppb lead, 1 ppb iron, .05 ppb bismuth. With all allowable impurities at these low levels, there is a reasonable argument for not being concerned. Still, sometimes small things make a big
difference.
99.99% silver (.9999 fine) has total allowable impurities of 100 ppm of the same metals in the
same ratios, and costs (in wire form) between $50-$90 above the spot price of silver.
99.999% silver (.99999 fine) has total allowable impurities of 10 ppm, and in wire form costs
about $250 above the spot price. 99.999% silver, in wire form, costs more than gold and is
very difficult to find commercially.
We use 99.99% silver in producing our product. Click here for details.
References:
http://educate-yourself.org/cs/csarticle2.shtml
Agency for Toxic Substances and Disease Registry, on-line information service: (http://atsdr1.atsdr.cdc.gov:8080/ToxProfiles)
Kehoe, R. A. et al, 1940. J. Nutr. 19:579.
Kent, J.T. Lectures on Homeopathic Materia Medica, Jain Publishing Co. New Delhi, India, reprint 1982.
Michaelis, L. The Effects of Ions in Colloidal Systems, Williams & Williams Co. Baltimore, MD, 1925.
Ostwald, W. Practical Colloid Chemistry, Methuen & Co. Ltd. London, UK, 1926.
Simonetti, N. et al, Electrochemical Ag+ for Preservative Use. Applied and Environmental Microbiology. American Society for Microbiology: Washington,1992, Vol. 58, No. 12, pp 3834-3836.
Disclaimer: These products have not been tested or approved by the U.S. Food
and Drug Administration. These products are not intended to diagnose, prevent,
treat or cure any disease. KinetiTeK Nutraceutical Solutions makes no claims or promises as to health benefits of its dietary/health supplements. All research information is provided as a courtesy to our customers. Consequences of dietary, topical or other use of any product is the sole responsibility of the customer.
© 2017 All rights reserved. KinetiTeK is a registered trademark.